Fundamentals of Batteries

Batteries are electrochemical devices that store chemical energy and convert it into electrical energy. They are ubiquitous in our modern lives, powering everything from our smartphones and laptops to electric vehicles and grid-scale energy storage systems. Understanding the fundamental principles behind how batteries work is crucial for appreciating their capabilities and limitations.
Basic Principles of Battery Operation
Batteries function based on the movement of ions between two electrodes through an electrolyte. This movement of ions creates an electric current that can be used to power devices. The process involves two key reactions: a chemical reaction that releases electrons at the negative electrode (anode) and a chemical reaction that consumes electrons at the positive electrode (cathode). The difference in electrical potential between the two electrodes is known as the battery’s voltage.
Key Components of a Battery
- Electrodes: The electrodes are the conductive materials that participate in the chemical reactions. The anode is the negative electrode, where oxidation occurs, releasing electrons. The cathode is the positive electrode, where reduction occurs, consuming electrons.
- Electrolyte: The electrolyte is a solution or solid that allows ions to move between the electrodes. It can be a liquid, a solid, or a gel.
- Separator: The separator is a porous membrane that prevents the electrodes from touching, preventing a short circuit. It allows ions to pass through while blocking the flow of electrons.
- Case: The case encloses the battery and protects the internal components. It can be made of various materials, including metal, plastic, or ceramic.
Chemical Reactions in Battery Operation
The chemical reactions that occur within a battery are responsible for the storage and release of energy. These reactions involve the transfer of electrons between the electrodes and the electrolyte. The specific reactions vary depending on the type of battery.
For example, in a lithium-ion battery, the anode is typically made of graphite, and the cathode is made of a lithium-containing compound, such as lithium cobalt oxide. During discharge, lithium ions move from the anode to the cathode, releasing electrons that flow through an external circuit to power a device. During charging, the process is reversed, with lithium ions moving back to the anode.
The overall chemical reaction in a lithium-ion battery can be represented as:
LixC6 + Li1-xCoO2 ⇌ LiCoO2 + C6
Types of Batteries
Batteries are classified based on their chemical composition and operating principles. Each type has unique characteristics in terms of energy density, power density, cycle life, and cost. Here are some common types of batteries:
- Lead-acid batteries: These are the oldest and most widely used type of battery. They are relatively inexpensive and have high power density, making them suitable for applications such as car batteries and backup power systems. However, they have low energy density and a limited cycle life.
- Nickel-cadmium (NiCd) batteries: NiCd batteries are known for their durability and long cycle life. They were commonly used in portable electronics and power tools but have been largely replaced by lithium-ion batteries due to environmental concerns about cadmium.
- Nickel-metal hydride (NiMH) batteries: NiMH batteries offer higher energy density than NiCd batteries and are more environmentally friendly. They are often used in hybrid electric vehicles and consumer electronics.
- Lithium-ion batteries: Lithium-ion batteries have become the dominant battery technology in recent years. They offer high energy density, high power density, and long cycle life, making them ideal for applications such as smartphones, laptops, electric vehicles, and grid-scale energy storage.
- Lithium-sulfur batteries: Lithium-sulfur batteries have the potential for even higher energy density than lithium-ion batteries. However, they are still under development and face challenges related to cycle life and safety.
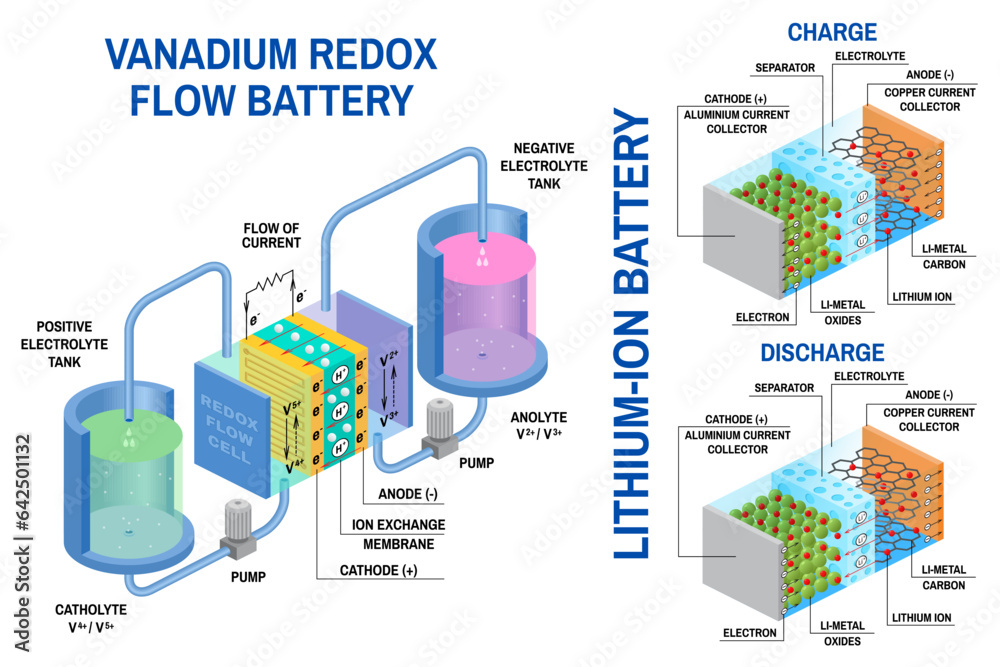
- Flow batteries: Flow batteries are different from other types of batteries in that they store energy in liquid electrolytes rather than solid electrodes. They have a long cycle life and are well-suited for grid-scale energy storage applications.
Battery Applications and Uses: What Is Battery
Batteries have become an indispensable part of modern life, powering everything from our smartphones and laptops to electric vehicles and renewable energy systems. Their versatility and ability to store energy make them crucial for various applications across different industries.
Everyday Applications
Batteries are ubiquitous in our daily lives, powering a wide range of devices and appliances. From small, portable electronics like smartphones, laptops, and cameras to larger appliances like power tools, electric bikes, and even medical devices, batteries play a vital role in our convenience and productivity.
- Portable Electronics: Batteries power our smartphones, laptops, tablets, and other portable devices, enabling us to stay connected and productive on the go.
- Household Appliances: Batteries power a wide range of household appliances, including cordless vacuum cleaners, electric toothbrushes, shavers, and remote controls, offering convenience and flexibility.
- Toys and Games: Batteries power toys, games, and other recreational devices, providing entertainment and fun for children and adults alike.
- Medical Devices: Batteries power life-saving medical devices such as pacemakers, insulin pumps, and hearing aids, improving the quality of life for patients.
Industrial Applications
Beyond everyday applications, batteries play a crucial role in various industries, enabling efficient operations and advancements in technology.
- Electric Vehicles: Batteries are the heart of electric vehicles (EVs), storing energy that powers the motors and enabling zero-emission transportation.
- Renewable Energy Systems: Batteries store energy generated from renewable sources like solar and wind power, ensuring a consistent and reliable energy supply.
- Power Backup Systems: Batteries provide reliable power backup in case of power outages, ensuring the continued operation of critical systems in homes, businesses, and hospitals.
- Grid-Scale Energy Storage: Batteries play a vital role in grid-scale energy storage, helping to balance energy supply and demand and improve the reliability of power grids.
Advantages and Disadvantages
Batteries offer numerous advantages, making them suitable for various applications. However, they also have certain disadvantages that need to be considered.
Advantages:
- Portability: Batteries are compact and lightweight, making them ideal for portable devices and applications.
- Energy Storage: Batteries can store energy efficiently, enabling the use of electricity in places where it’s not readily available.
- Clean Energy: Batteries can be used to store energy from renewable sources, contributing to a cleaner and more sustainable energy future.
- Long Lifespan: Modern batteries have a relatively long lifespan, offering extended use and reducing the need for frequent replacements.
Disadvantages:
- Limited Capacity: Batteries have a limited capacity, meaning they can only store a certain amount of energy before needing to be recharged.
- Charging Time: Recharging batteries can take time, which can be a drawback in situations where immediate power is required.
- Environmental Concerns: Battery production and disposal can have environmental impacts, particularly related to the extraction of raw materials and the management of hazardous waste.
- Cost: Batteries can be relatively expensive, especially for larger applications like electric vehicles and grid-scale energy storage.
Electric Vehicles
Electric vehicles (EVs) are powered by batteries, which store energy that drives the electric motors. EVs offer numerous advantages over traditional gasoline-powered vehicles, including:
- Zero Emissions: EVs produce no tailpipe emissions, contributing to cleaner air and reduced greenhouse gas emissions.
- Energy Efficiency: EVs are more energy-efficient than gasoline-powered vehicles, meaning they can travel further on a single charge.
- Quiet Operation: EVs are significantly quieter than gasoline-powered vehicles, reducing noise pollution in urban areas.
- Lower Maintenance Costs: EVs require less maintenance than gasoline-powered vehicles, as they have fewer moving parts.
Renewable Energy Systems, What is battery
Batteries play a crucial role in renewable energy systems, storing energy generated from sources like solar and wind power. This enables a consistent and reliable energy supply, even when the sun is not shining or the wind is not blowing.
- Solar Energy Storage: Batteries store excess solar energy generated during the day, allowing it to be used at night or during periods of low sunlight.
- Wind Energy Storage: Batteries store energy generated by wind turbines, ensuring a continuous energy supply even when the wind is not blowing consistently.
- Off-Grid Power Systems: Batteries power off-grid systems in remote areas or for homes seeking energy independence.
Innovative Battery Technologies
Researchers and engineers are continuously developing innovative battery technologies to improve performance, reduce costs, and address environmental concerns.
- Lithium-ion Batteries: Lithium-ion batteries are currently the most widely used type of battery, offering high energy density and long lifespan.
- Solid-State Batteries: Solid-state batteries are a promising technology that uses solid electrolytes instead of liquid electrolytes, potentially offering higher energy density, faster charging, and improved safety.
- Flow Batteries: Flow batteries store energy in liquid electrolytes, enabling large-scale energy storage and long discharge durations.
- Redox Flow Batteries: Redox flow batteries use redox reactions to store energy, offering high capacity and long cycle life.
Battery Performance and Characteristics

A battery’s performance is measured by several key metrics that determine its suitability for a particular application. These metrics provide insights into how effectively a battery stores and delivers energy, its longevity, and its overall efficiency.
Key Performance Metrics
Battery performance is evaluated based on several crucial metrics:
- Capacity: The amount of electrical charge a battery can store, typically measured in Ampere-hours (Ah) or milliampere-hours (mAh). A higher capacity indicates a battery can deliver power for a longer duration. For example, a 100 Ah battery can supply 1 Ampere of current for 100 hours or 100 Amperes for 1 hour.
- Voltage: The electrical potential difference between the battery’s positive and negative terminals, measured in Volts (V). Voltage determines the force with which the battery pushes electrons through a circuit. For instance, a 12V battery provides a higher voltage than a 3.7V battery, resulting in more powerful operation.
- Current: The rate at which electrical charge flows through a circuit, measured in Amperes (A). Current is directly proportional to the power delivered by the battery. A higher current rating implies a battery can deliver more power to the connected device.
- Lifespan: The number of charge-discharge cycles a battery can endure before its performance significantly degrades. Lifespan is influenced by factors like charging and discharging rates, operating temperature, and depth of discharge. Batteries with longer lifespans are preferred for applications requiring frequent use or extended operation.
Factors Affecting Battery Performance
Several factors can significantly impact a battery’s performance, influencing its efficiency and longevity:
- Temperature: Extreme temperatures, both high and low, can negatively affect battery performance. High temperatures accelerate chemical reactions within the battery, leading to faster degradation and reduced lifespan. Conversely, low temperatures can hinder chemical reactions, reducing capacity and increasing internal resistance. Maintaining optimal operating temperatures is crucial for maximizing battery performance and lifespan.
- Charging Rate: The speed at which a battery is charged can influence its performance and lifespan. Fast charging can generate heat within the battery, potentially leading to degradation. Slow charging, while extending the charging time, generally results in less stress on the battery and a longer lifespan. The optimal charging rate depends on the specific battery chemistry and application requirements.
- Discharge Rate: The rate at which a battery delivers energy to a load affects its performance. High discharge rates can lead to increased internal resistance and voltage drop, reducing the available power. Lower discharge rates generally result in higher efficiency and longer operating time. The ideal discharge rate is determined by the specific application and the battery’s capacity and current rating.
Battery Degradation
Battery degradation is a gradual process that reduces a battery’s capacity, power output, and lifespan. It is a natural phenomenon that occurs over time due to various factors:
- Electrolyte Decomposition: The electrolyte, a liquid or gel that facilitates ion movement within the battery, can decompose over time, leading to reduced capacity and increased internal resistance.
- Electrode Degradation: The electrodes, which store and release ions during charge and discharge cycles, can degrade due to factors like mechanical stress, chemical reactions, and formation of dendrites. Electrode degradation reduces the battery’s capacity and power output.
- Formation of Solid Electrolyte Interphase (SEI): A protective layer forms on the anode surface during the first few charge cycles. This layer, called the SEI, can grow over time, increasing internal resistance and reducing capacity.
- Internal Resistance: Internal resistance increases as the battery ages, resulting in reduced power output and increased heat generation. This can be attributed to factors like electrode degradation, electrolyte decomposition, and the formation of SEI.
Battery Performance Testing and Evaluation
Various methods are employed to test and evaluate battery performance:
- Capacity Test: This test measures the battery’s ability to store and deliver electrical charge. It involves fully charging the battery and then discharging it at a constant current until a predetermined voltage threshold is reached. The capacity is calculated by integrating the current over the discharge time.
- Voltage Test: This test measures the battery’s voltage under various load conditions. It provides insights into the battery’s ability to maintain a stable voltage output during operation.
- Current Test: This test measures the battery’s ability to deliver current at different discharge rates. It helps determine the battery’s power output capabilities and its suitability for various applications.
- Lifespan Test: This test simulates the battery’s use over time by subjecting it to repeated charge and discharge cycles. It helps determine the battery’s lifespan and its ability to maintain performance over extended use.
- Internal Resistance Test: This test measures the internal resistance of the battery, which indicates the battery’s health and its ability to deliver power efficiently.
- Temperature Test: This test evaluates the battery’s performance at different temperatures, providing insights into its tolerance to extreme conditions.
What is battery – A battery is a device that stores chemical energy and converts it into electrical energy. It’s a fundamental component of many modern technologies, from smartphones to electric vehicles. Speaking of modern technologies, it’s fascinating to see how young stars like Skai Jackson are using their platforms to advocate for important causes, like you can read about in skai jackson news.
Just like batteries power our devices, these young voices are powering positive change in the world. So, the next time you pick up your phone or plug in your car, remember the power of batteries and the power of young voices making a difference.
Battery, in legal terms, refers to the unlawful application of force upon another person. It’s a serious offense with varying degrees of severity, depending on the circumstances. The recent case of skai jackson jail highlights the complexities of such charges, where understanding the nuances of the law is crucial in determining culpability and potential consequences.
Regardless of the situation, it’s important to remember that battery is a crime that can have lasting impacts on the victim and the accused.